Category:2D materials
Every real crystal or material has a surface. In VASP simulations, however, periodic boundary conditions typically model an infinite crystal, the perfect bulk. To model a surface, a thin film, or an intrinsically 2D material in VASP, therefore, requires breaking these periodic boundary conditions intentionally. This is done by elongating the simulation cell in one direction (normal to the intended surface) without adding more atoms. This creates a vacuum region between repeated images of thin films of material (commonly called surface slabs, or just slabs), each of which has two surfaces.
| Mind: It is not possible to create only a single surface in an atomistic model with periodic boundary conditions. Cleaving a bulk material inevitably produces two surfaces. These two surfaces are generally nonequivalent, although they may be identical depending on the material and the cleavage plane. |
Nomenclature
Surfaces
As discussed above, in atomistic simulations employing periodic boundary conditions, surfaces are modeled as thin films of a material separated by a vacuum region of sufficient thickness. When the surface itself is the primary focus, the slab model must be constructed to mimic a semi-infinite bulk crystal beneath the surface. The second surface, located on the opposite side of the slab, should be sufficiently separated to prevent strong interactions between the two surfaces.
Thin films
Although thin-film simulations often employ the same computational setup and slab model as surface calculations, their objective differs. In thin-film studies, the goal is to investigate the behavior and properties of the entire system — including both surfaces and the bulk-like interior region — rather than isolating the characteristics of a single surface.
2D materials
This class of materials comprises ultrathin films consisting of only a few atomic layers. Prototypical examples include graphene, which is one atomic layer thick, and molybdenum disulfide (MoS$_2$), which consists of three atomic layers. Such materials can occur naturally as van der Waals–bonded layered crystals, but they can also be exfoliated and studied as mono-, bi-, or few-layer systems.
Symmetry and stoichiometry
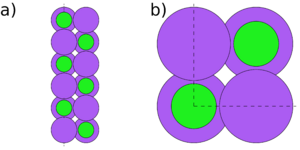
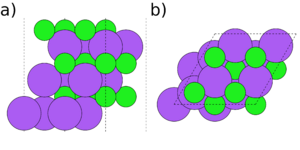
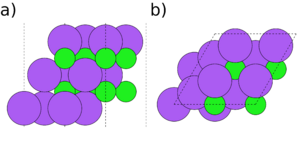
Symmetry and stoichiometry are important concepts for surface slabs. Figs. 1 to 3 show three different surface slabs of NaCl, a 001 and two 111 slabs. All 001 lattice planes contain an equal amount of Na and Cl atoms, so slabs of all thicknesses are both symmetric (both surfaces are equivalent) and stoichiometric (the bulk ratio 1:1 of the atom types is preserved in the slab). The 111 lattice planes contain either Na or Cl. Thus, the slabs can either be stoichiometric (Fig. 2) or symmetric (Fig. 3), but it is impossible to achieve both.
Symmetry
If a slab is non-symmetric, it is important to consider dipole corrections. The non-symmetric 111 NaCl slab (Fig. 2) is forming a dipole, with $Cl^{-}$ and $Na^{+}$ terminations on opposite sides of the slab. This leads to a potential gradient through the vacuum, which is not physical and leads to extra interaction between the slab replicas. In general, this can be true for any non-symmetric slabs, not only for ionic crystals like NaCl. In those cases, dipole corrections should always be turned on and the size of the dipole checked in the OUTCAR file. However, energy differences due to dipole interactions are usually smaller than energy differences due to a different exchange-correlation functional. They can also interfere with convergence during relaxations. It is thus usually advisable to initially relax the system without dipole corrections and then turn on the corrections in a static follow-up calculation and proceed based on the size of the effect.
Slab symmetry is also important for surface calculations. If the two surfaces of a slab are not equal, the computation of the surface energy becomes more cumbersome.
Stoichiometry
Slab stoichiometry is usually less important than symmetry for the computation setup. But in surface energy calculation methods that reference the bulk energy (which is always stoichiometric), complications can arise.
Advice and recommendations
Creating slab models
A clean surface is defined uniquely by the bulk crystal, the lattice plane where the cut should be placed, and the termination of the surface. For a slab model, additional parameters are important, mainly the slab thickness and the thickness of the vacuum region. Both need to be converged independently of each other to be sure to get accurate results.
While it is entirely possible to create a slab model by visualizing a crystal and thinking hard about lattice planes and stacking, it is usually more convenient and less error-prone to use available tools. The ASE[1] and pymatgen[2] both provide surface building capabilities. The following ASE code snippet creates a NaCl 001 slab with 6 layers and 10 Å vacuum, for example:
from ase.build import bulk, surface
from ase.io import write
nacl_bulk = bulk('NaCl', 'rocksalt', a=5.64, cubic=True)
slab_001 = surface(nacl_bulk, (0,0,1), 3)
slab_001.center(vacuum=10.0, axis=2)
write('POSCAR_NaCl_001_6layers.vasp', slab_001, format='vasp', direct=True, sort=True)
In-plane lattice parameters
When two surfaces are created by cleaving a crystal, bonds are broken, and energy needs to be spent. This energy is called surface energy, and it can differ significantly between different lattice planes and surface terminations. In real materials, the surface energy is vanishingly small compared to the bonding energy in the bulk crystal, but for a surface slab model, it can be comparatively large. Thus, the in-plane lattice parameter will usually shrink compared to the bulk crystal and depend on the number of layers in the slab.
| Tip: For surface calculations, it is prudent to use the in-plane lattice parameter of the bulk, and not relax it, since the surface of a semi-infinite bulk would retain the bulk lattice parameter as well. For a thin film calculation, however, the in-plane lattice parameters should be relaxed to relieve in-plane strain. |
Out-of-plane lattice parameters
Since bonds will be cut when a surface is created, the spacing between the first two layers of the surface will usually relax towards each other, with respect to the bulk lattice spacing. This effect is often important for the surface properties and should not be disregarded. Avoiding this relaxation on the slab side opposite to the surface of interest, however, can reduce the total number of layers needed to mimic the semi-infinite bulk.
| Tip: For a surface calculation, it is often beneficial to fix several layers at the bottom of the slab to simulate the semi-infinite bulk more efficiently and with fewer total layers. The Selective Dynamics options in the POSCAR file can be used for this purpose. A thin film should be fully relaxed. |
Surface energy calculations
The surface energy $\gamma$ is an important characteristic of any surface, determining its stability and influencing its adhesion properties, catalytic ability, and ability to form thin films and interfaces.
For a symmetric and stoichiometric surface slab, the surface energy $\gamma$ of the two equivalent surfaces is given by: \begin{equation} \gamma = \frac{1}{2A}\left[E_\mathrm{slab} - N E_\mathrm{bulk}\right] \quad, \end{equation} where $E_\mathrm{slab}$ is the total energy of the slab, $E_\mathrm{bulk}$ is the total energy of the bulk, and $N$ is the multiplicity of formula units in the slab compared to those in the bulk cell.
A more general formula, that holds for non-stoichiometric and non-symmetric slabs for the surface energies $\gamma_1$ and $\gamma_2$ on both sides of the slab is \begin{equation} \gamma_1 = \frac{1}{A}\left[E_\mathrm{slab} - \sum_i N_i\mu_i(p, T)\right] - \gamma_2 \quad, \label{eqn:gen_surfen} \end{equation} where the bulk energy is replaced by a therm with the chemical potentials $\mu_i$, which influence the surface energies. Thus, the value of the temperature- and pressure-dependent chemical potential can determine which surface is energetically most favourable.
| Tip: The surface energy needs to be converged with respect to the number of layers and the vacuum thickness. This can be tricky. To get good convergence of the surface energy with respect to the number of layers, it is beneficial to use a bulk cell with the same symmetry as the surface cell, to ensure equivalent k-point sampling. |
Please also consult also our on Ni(111) for some more in-depth information about surface energy calculations.
Related pages
References
Pages in category "2D materials"
This category contains only the following page.
